 Image 1 of 1
Image 1 of 1


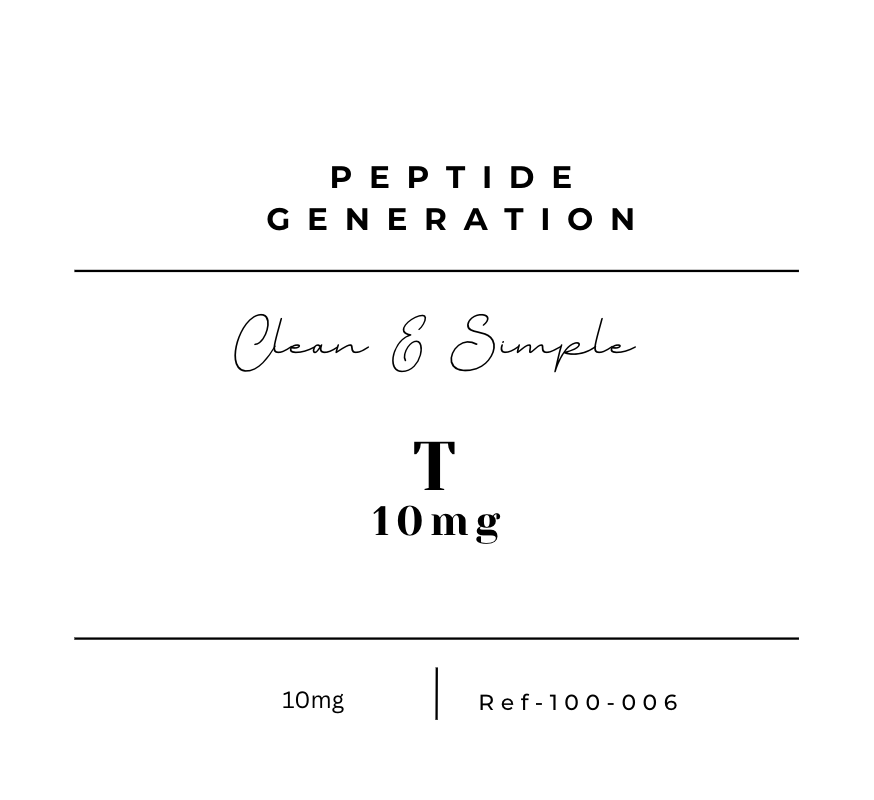
T 10mg
🔬 What Is Tirzepatide?
Tirzepatide is a dual incretin receptor agonist that activates both:
GLP-1 receptor → reduces appetite, slows gastric emptying, improves insulin secretion.
GIP receptor → enhances insulin sensitivity, may reduce GI side effects of GLP-1s.
Brand names:
Mounjaro → FDA-approved for type 2 diabetes (2022).
Zepbound → FDA-approved for chronic weight management (2023).
📊 Clinical Benefits
Type 2 Diabetes
Lowers A1C by 2–2.5% (greater than semaglutide in head-to-head trials).
Improves fasting glucose, post-meal glucose, and insulin sensitivity.
Obesity / Weight Loss
In SURMOUNT trials, average weight loss at 72 weeks:
5 mg → ~15%
10 mg → ~19.5%
15 mg → ~21%
Some individuals lost >25% of body weight, approaching bariatric surgery outcomes.
Other Benefits (under study)
Reduces visceral and liver fat (promising for NAFLD/NASH).
Improves blood pressure, lipids, and markers of cardiovascular health.
Being evaluated in cardiovascular outcomes trials and sleep apnea trials.
⚠️ Side Effects
Most common:
Nausea, vomiting, diarrhea, constipation, decreased appetite.
Other considerations:Gallbladder disease risk (like GLP-1s).
Rare pancreatitis or GI obstruction.
Small risk of thyroid C-cell tumors (contraindicated in patients with personal/family history of medullary thyroid carcinoma or MEN2).
✅ Key Takeaways
Tirzepatide = dual GLP-1 + GIP agonist.
Approved as Mounjaro (T2D) and Zepbound (obesity).
Efficacy: Among the most potent incretin therapies → up to 21% body weight loss.
Dose: Weekly injections, titrated from 2.5 → up to 15 mg.
Benefits: Improves weight, blood sugar, insulin resistance, liver fat, and heart health markers.
Side effects: Mostly GI, plus potential long-term thyroid/gallbladder concerns.
🔬 What Is Tirzepatide?
Tirzepatide is a dual incretin receptor agonist that activates both:
GLP-1 receptor → reduces appetite, slows gastric emptying, improves insulin secretion.
GIP receptor → enhances insulin sensitivity, may reduce GI side effects of GLP-1s.
Brand names:
Mounjaro → FDA-approved for type 2 diabetes (2022).
Zepbound → FDA-approved for chronic weight management (2023).
📊 Clinical Benefits
Type 2 Diabetes
Lowers A1C by 2–2.5% (greater than semaglutide in head-to-head trials).
Improves fasting glucose, post-meal glucose, and insulin sensitivity.
Obesity / Weight Loss
In SURMOUNT trials, average weight loss at 72 weeks:
5 mg → ~15%
10 mg → ~19.5%
15 mg → ~21%
Some individuals lost >25% of body weight, approaching bariatric surgery outcomes.
Other Benefits (under study)
Reduces visceral and liver fat (promising for NAFLD/NASH).
Improves blood pressure, lipids, and markers of cardiovascular health.
Being evaluated in cardiovascular outcomes trials and sleep apnea trials.
⚠️ Side Effects
Most common:
Nausea, vomiting, diarrhea, constipation, decreased appetite.
Other considerations:Gallbladder disease risk (like GLP-1s).
Rare pancreatitis or GI obstruction.
Small risk of thyroid C-cell tumors (contraindicated in patients with personal/family history of medullary thyroid carcinoma or MEN2).
✅ Key Takeaways
Tirzepatide = dual GLP-1 + GIP agonist.
Approved as Mounjaro (T2D) and Zepbound (obesity).
Efficacy: Among the most potent incretin therapies → up to 21% body weight loss.
Dose: Weekly injections, titrated from 2.5 → up to 15 mg.
Benefits: Improves weight, blood sugar, insulin resistance, liver fat, and heart health markers.
Side effects: Mostly GI, plus potential long-term thyroid/gallbladder concerns.

